Positive Impact of Dragonfly™ Discovery in Biochemical Assay
Biochemical assays are commonly used to assess how organic compounds impact the activity of a protein of interest (POI). ZoBio frequently employs biochemical assays to validate compounds that have been identified as interacting with the POI through orthogonal biophysical assays like Surface Plasmon Resonance (SPR) and Nuclear Magnetic Resonance (NMR). To enhance the efficiency and reliability of the biochemical assay workflow, ZoBio has purchased a Dragonfly™ Discovery, a valuable tool known for its ability to significantly improve assay performance by facilitating high-throughput, accurate, and programmable liquid dispensing. In the example below, we demonstrate how the Dragonfly™ Discovery allowed us to execute a challenging assay with accuracy in a high throughput manner. Furthermore, the capability to dispense multiple reagents, makes the instrument suitable for complex studies of the Mode of Action of inhibitors.
The ADP-Glo™ lipid kinase assay is a biochemical assay designed to detect the phosphorylation of a lipid substrate by lipid kinases. We used this kit to characterize the IC50 of inhibitors for a class I lipid kinase, PI3Kα which phosphorylates PIP2 to generate PIP3. In order to be cost-effective, the kinase reaction had to be performed in a small, 6 µl volume in a 384-wellplate. Each assay component including the compound to be tested, PI3Kα, ATP and the PIP2 substrate needed to be dispensed accurately into the wells in volumes ranging from 1 to 2 µl to initiate the kinase reaction. After an incubation period, the reaction was quenched and eventually the accumulated ADP was converted into luminescence light by addition of ADP-Glo detection solutions. Our existing 384-channel pipetting robots faced limitations in handling small volumes accurately. The result was a need for manual dispensing and mixing of components, which significantly impacted the efficiency and reliability of the assay. Our researchers could process a maximum of only 15 compounds per day, making the process time-consuming and inefficient for data collection. The manual pipetting, especially when dealing with the viscous PIP2 substrate solution, led to poor data reproducibility. This inconsistency in the pipetting process introduced errors and variability in the assay results.
To address these challenges and improve the assay workflow, ZoBio scientists integrated the Dragonfly™ Discovery into their processes. This sample handling automation resulted in a several significant improvements. The scientists were capable of dispensing reagents at high speed, taking less than 1 minute to process a 384-well plate while significantly reducing variation between wells. This speed and accuracy even extended to the viscous lipid containing solutions needed for PI3ka activity. The assay throughput was improved significantly without compromising the assay performance as indicated by a Z' value greater than 0.8, which is a common measure of assay quality, indicating the suitability of the assay for high-throughput screening (Figure 1). The introduction of the Dragonfly™ Discovery significantly enhanced the throughput of the assay, while at the same time reducing the need for replicate measurements. This not only saved time but also minimized reagent consumption and lowered the overall cost per assay point.
Figure 1. Lipid kinase ADP-Glo workflow A) samples dispensed by handheld electronic pipettes and B) samples dispensed by Dragonfly™ Discovery.
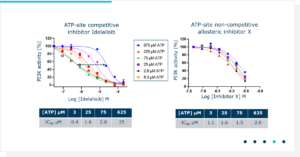
Our Dragonfly’s flexibility allows us to prepare complicated assays since it can pipette up to 10 different components. Since we sought allosteric inhibitors of PI3Kα, it was important to validate this Mode of Action for biophysically selected hits using an enzyme assay that monitors the IC50 of an inhibitor while increasing the concentration of ATP in the lipid kinase assay (Figure 2). Various concentrations of ATP were prepared by Dragonfly while keeping the compound concentration range constant. Idelalisib, a well characterized ATP site inhibitor, bound apo-PI3Kα with a KD of 0.7 µM in SPR. The IC50 of Idelalisib shifted from 0.4 to 25 µM as the concentration of ATP increased from 3 to 625 µM, confirming its ATP competitive mechanism. In contrast, the IC50 of Inhibitor X did not significantly change as the ATP concentration increased, suggesting an allosteric mechanism. This mechanism of compound X was orthogonally confirmed using SPR where binding was shown to be non-competitive with Idelalilsib.
Figure 2. Determination of the mode of action of PI3Kα inhibitors.
Stay tuned for insights, discoveries, and a closer look at the transformative role of technology in shaping our research endeavors.